top of page

María Arufe
UDC Professor
Senescence Research Lines
ENDOMETRIOSIS
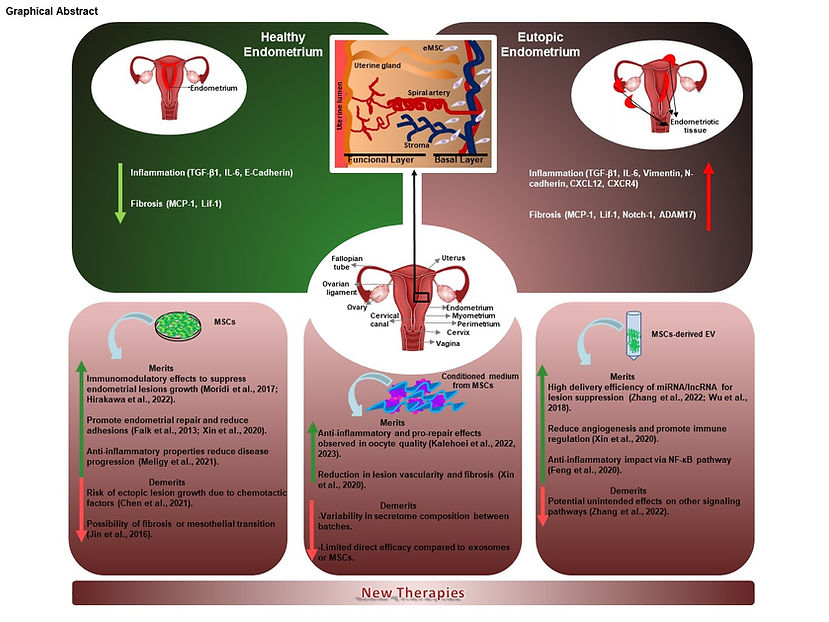
Endometriosis is a complex gynaecological condition that remains difficult to treat, largely because of delayed diagnosis and the wide variability of its symptoms. Mesenchymal stem cell (MSC) therapies and their derivatives, such as modified extracellular vesicles and conditioned media, have emerged as promising therapeutic strategies, acting directly on the proliferative and inflammatory processes central to the disease. Understanding current advances in experimental treatments involving stem cells and their derivatives is therefore of critical importance. A comprehensive review of MSC-based therapies in endometriosis is essential not only to consolidate existing knowledge and identify research gaps but also to evaluate the safety and efficacy of these approaches. Such a review may contribute to the development of standardised protocols and regulatory frameworks, supporting the progression from experimental models to clinical application. Ultimately, this could accelerate the development of innovative, disease-modifying therapies, offering hope to those affected by the debilitating impact of endometriosis. This review primarily explores the molecular mechanisms underlying inflammation in endometriosis and highlights experimental therapies involving MSCs and their derivatives. Particular focus is given to the therapeutic potential of MSCs in both cell- and gene-based interventions, with an emphasis on the molecular pathways involved in tissue repair and healing.
Collaboration with Dr. López-Viñas, Dr. Quintana & Dr. Guillán
HUTCHINSON-GILFORD PROGERIA SYNDROME (HGPS)
Hutchinson-Gilford Progeria Syndrome (HGPS) is a fatal childhood disorder, which is considered a very rare disease. It is caused by an autosomal dominant mutation in the LMNA gene and is characterized by accelerated aging. Our group studies in parallel human cell lines from HGPS patients and healthy parental controls as well as human umbilical cord stem cells genetically modified to over-express LMNA and Progerin, using next-generation sequencing (NGS) and shotgun proteomic to unravel new molecular pathways. not previously altered. We have also developed an in vivo model of HGPS in zebrafish.
Collaboration with Dr. Carrera
Collaboration with Dr. Folgueira

INFLAMM-AGING
Mesenchymal stem cells have an important potential in the treatment of age-related diseases. In the last years, small extracellular vesicles derived from these stem cells have been proposed as cell-free therapies. Cellular senescence and proinflammatory activation are involved in the loss of therapeutic capacity and in the phenomenon called inflamm-aging. The regulators of these two biological processes in mesenchymal stem cells are not well-known. We found that p65 is activated during cellular senescence and inflammatory activation in human umbilical cord-derived mesenchymal stem cell. To demonstrate the central role of p65 in these two processes, we used small-molecular inhibitors of p65, such as JSH-23, MG-132 and curcumin. We found that the inhibition of p65 prevents the cellular senescence phenotype in human umbilical cord-derived mesenchymal stem cells. Besides, p65 inhibition produced the inactivation of proinflammatory molecules as components of a senescence-associated secretory phenotype (SASP) (interleukin-6 and interleukin-8 (IL-6 and IL-8)). Additionally, we found that the inhibition of p65 prevents the transmission of paracrine senescence between mesenchymal stem cells and the proinflammatory message through small extracellular vesicles. Our work highlights the important role of p65 and its inhibition to restore the loss of functionality of small extracellular vesicles from senescent mesenchymal stem cells and their inflamm-aging signature.

CELL FREE THERAPY
Workflow of the realization of the animal model of OA by injecting cells into the joint cavity of the left knee of the animals, which produces an increase in serum cytokines and chemokines in the animals in addition to the increase in SASP and markers of inflammation. Inhibition of miR-21 in MSCs, from the stroma of the human umbilical cord, by lentivirus and extraction of their EVs by ultracentrifugation. Finally, application of MSC therapy with its miR-21 inhibited or its EVs produces a decrease in serum cytokines and chemokines in the treated animals, in addition to an increase in SASP and markers of inflammation. The cell-free therapy being the one that produces a greater decrease in the parameters studied
Collaboration with Dr. Concha Gil
Collaboration with Dr. Mónica Carrera
Collaboration with Dr. Jesús Mateos

bottom of page